
A better way to secure data: Introducing Federated SSO to benefit Hospital IT
Implementing Federated Single Sign-On (SSO) simplifies authentication for MDIP users. This streamlined experience helps reduce login fatigue.

Latest release of Philips Capsule Surveillance receives FDA clearance

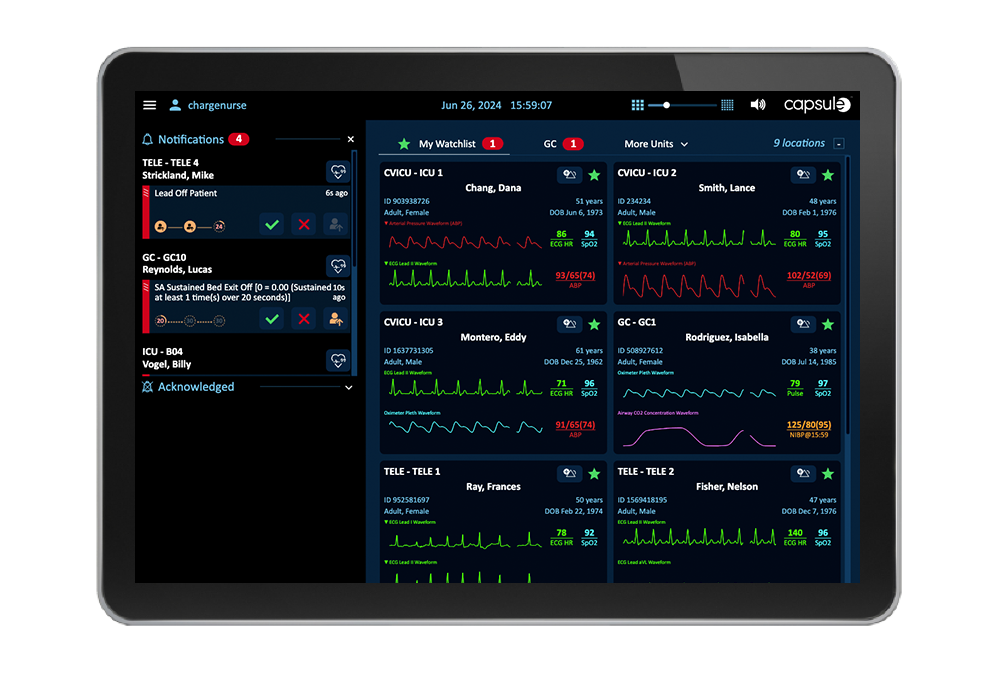
High profile alerts and alarms from all patient- connected bedside devices and systems are sent to the assigned care team in priority fashion. Alerts are filtered to reduce the number of alerts being propagated to avoid worsening alarm fatigue.
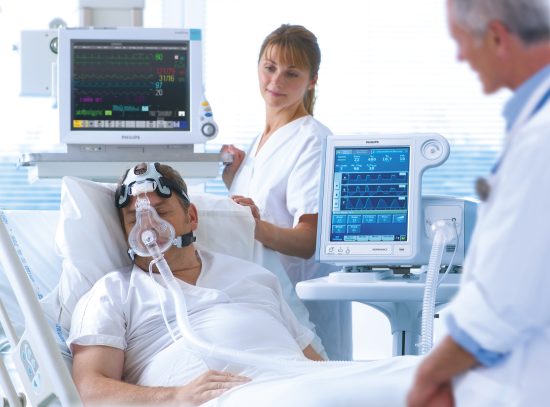
Tailored solution to support specialty trained nurses and caregivers to ensure that they can receive alarms and alerts from specialty devices and review live streaming patient data when they cannot be at the bedside.

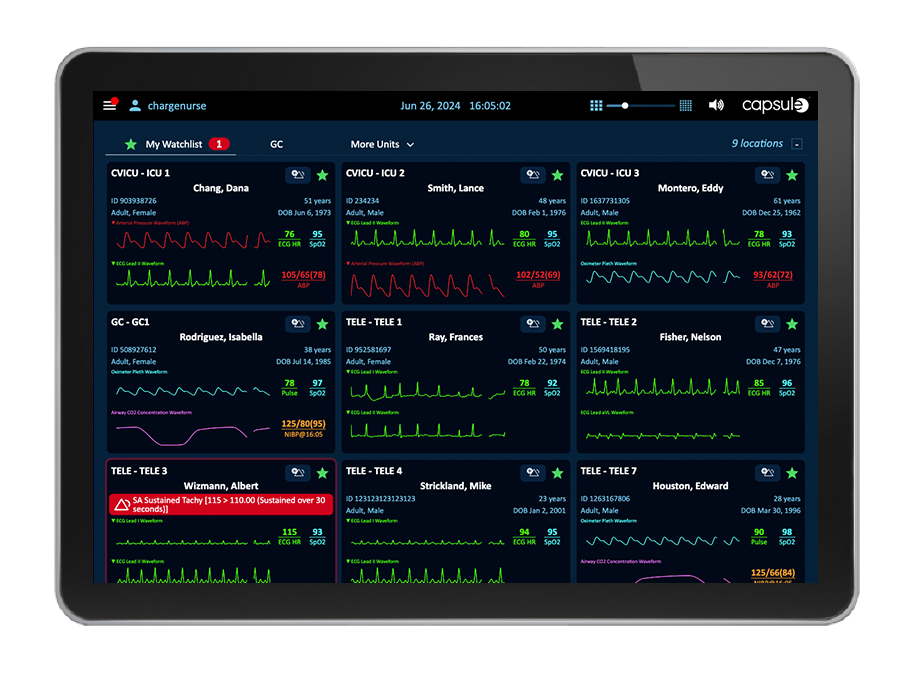
Visibility to all data, alarms, and alerts from patient-connected bedside devices and systems is critical to allow remote caregivers to see the patients clinical picture when not at the bedside.

Support caregivers where nurse to patient ratios are low with a way to watch for possible unnoticed decline and alert for early signs of respiratory distress.

Support the telemetry technician in the central monitoring unit with holistic visualization of all device and waveform data, enhanced alert escalation visibility and management, manual alert capabilities, and ECG strip annotation and export.
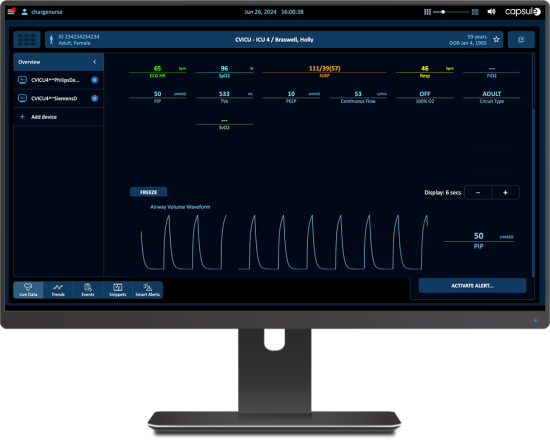
Continuous clinical surveillance can help extend the reach of frontline clinical staff over the full range of intensive care patients, regardless of where they receive care in the facility. Ventilated Patient Surveillance provides a centralized view of ventilator data and annunciates clinically actionable emergent events:
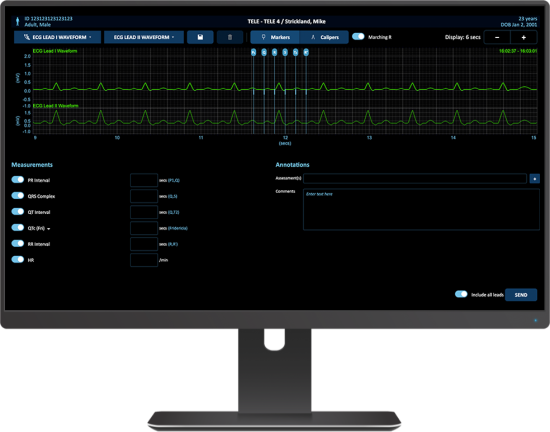
Capsule Surveillance provides comprehensive support for the telemetry central monitoring unit (CMU), and the associated workflows of the telemetry technician, by providing holistic visibility of all device and waveform data, enhanced alert escalation visibility and management, manual alert capabilities, and ECG strip annotation and export.

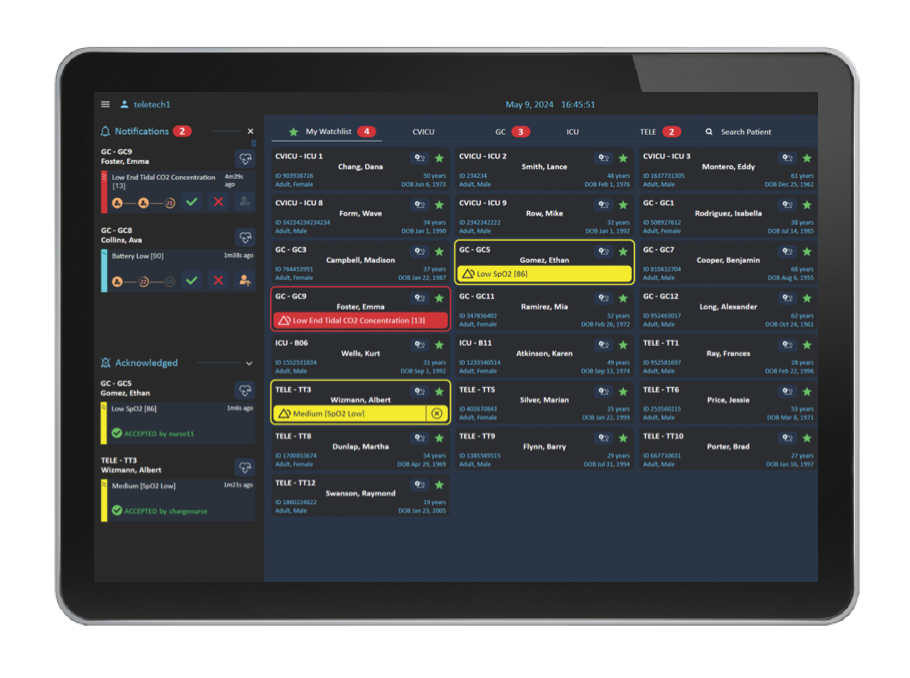
Telemetry techs need to ensure communication of clinically actionably alerts with frontline caregivers. Using the Alert Management Dashboard tele techs can track escalations of alerts and escalate or generate manual alerts as necessary. In one location, they can track, escalate, or generate manual alerts.
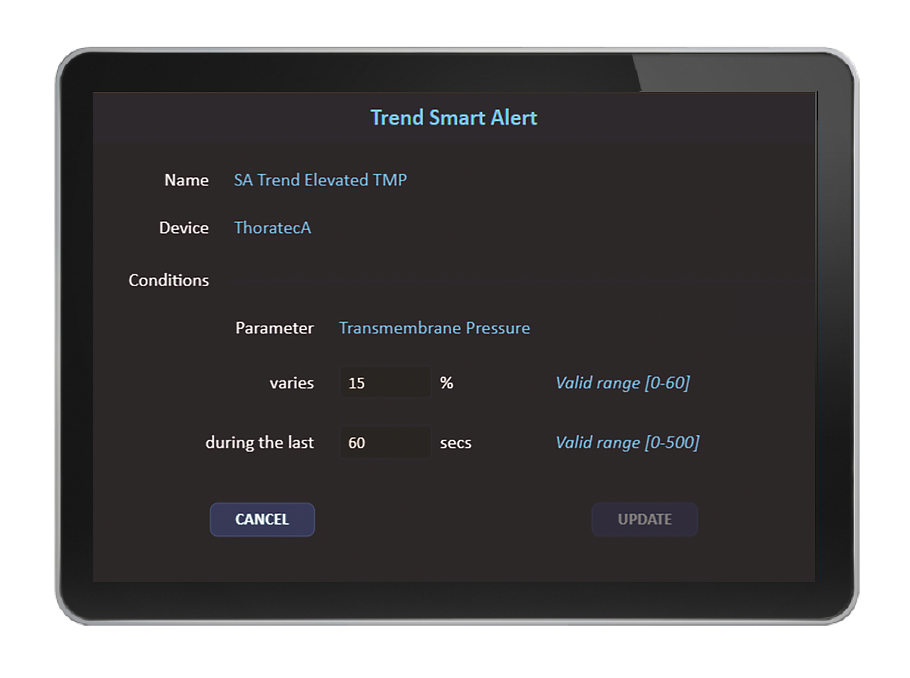
As part of implementation, Capsule specialists can work with clinical staff to configure Smart Rules for the facility and specific care areas. Capsule Surveillance allows for rules customization aligned with clinician preferences and practices. Certain Capsule Smart Rules support established, published clinical practice guidelines.
Through Smart Rules, Capsule Surveillance performs a multi-variable assessment of a patient and notifies caregivers with smart alerts when the patient’s condition shows signs of deterioration that can potentially lead to emergent events. Through smart alert distribution to the assigned care team members’ mobile devices or other remote screens, these notifications are made available to caregivers on the go or those who provide additional oversight, such as in an eICU, virtual care setting, or central monitoring unit.









Implementing Federated Single Sign-On (SSO) simplifies authentication for MDIP users. This streamlined experience helps reduce login fatigue.

EDM-enabled data is easier to use across a health system’s ecosystem, whether data is feeding into an EMR, central station, clinical decision support system, or an analytics platform.