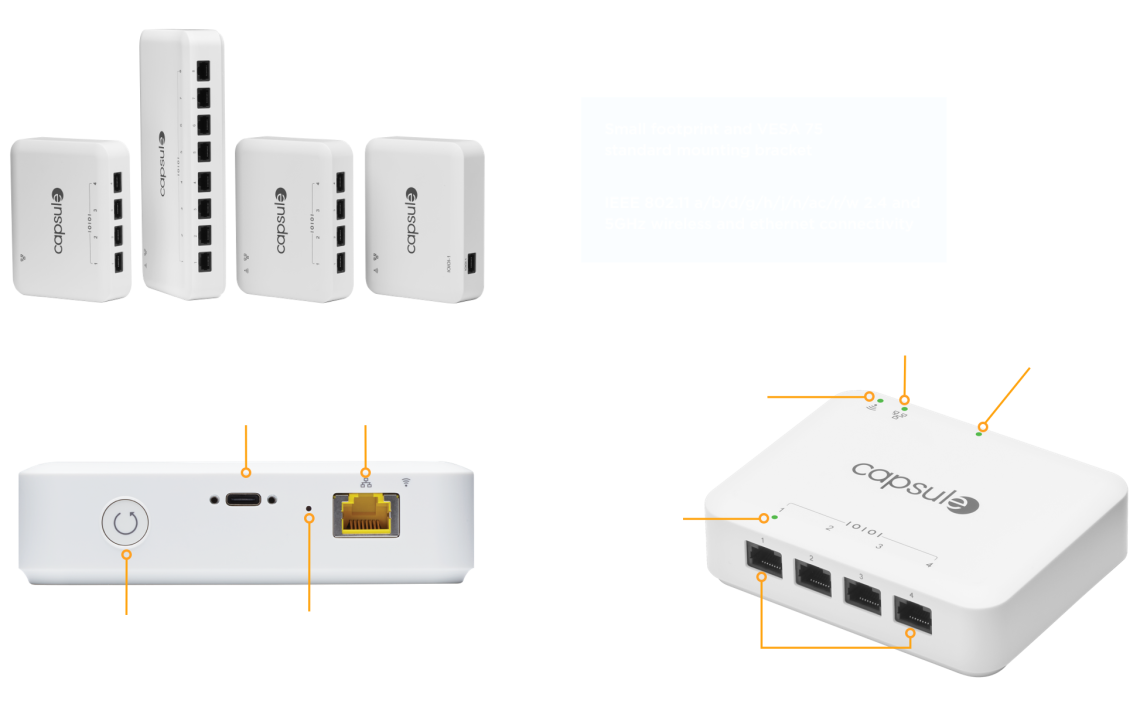
Compact, robust and reliable serial-to-network bridges
Philips Axon is the next generation of reliable, serial-to-network bridges within the Philips Capsule Medical Device Information Platform (MDIP), enabling the automatic collection of medical device data. The Philips Axon features an always-on design and connects to the hospital network through a wired Ethernet or standard 802.11 a/b/d/g/h/j/n/ ac/r/w wireless network.
Convenient and powerful options for any care area
Flexible configurations to meet your needs
- One, four, or eight port configurations to fit a wide range of use cases
- Support for both wired and wireless network connections to support stationary or mobile care use cases
- Small profile design enabling deployment in crowded environments
Easy and simple hardware deployment
- Power over ethernet offers the possibility for a single cable to support both power and network connection
- True medical device plug and play integration
Reliable connectivity and data integration
- Enables the integration of device data into the hospital EMR and other downstream systems
- Brings “always-on” data feeds to support patient surveillance capabilities to care areas with minimal space
- Expands access to data-driven interventions and precision care protocols in more areas of the hospital
Take a closer look at the Philips Axon and find out how it can help you deploy device integration with ease and optimize workflows.
Are you ready to advance
your care delivery?