Patient care increasingly depends on data streaming from bedside medical devices – such as monitors, ventilators, and infusion pumps – from a range of different manufacturers. Despite growing recognition of the importance of device connectivity, 94% of healthcare leaders say their organization experiences data integration challenges that impact its ability to provide timely, high-quality care, according to the Philips Future Health Index (FHI) 2024 Global Report.1 Fragmented data, inconsistent formats, and labor-intensive customizations are still slowing clinical workflows, complicating IT operations and limiting a hospital care team’s ability, at some degree, to act quickly.
Health systems need more interoperability. And at the heart of interoperability is Device Driver Interfaces (DDIs).
What Are DDIs and Why Do They Matter?
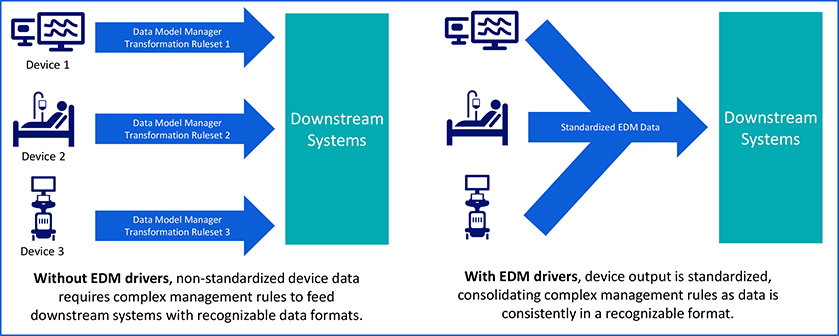
DDI’s are the backbone of device integration. They are pieces of software that act as a bridge between the medical device, such as a ventilator or monitor, and other systems, such as a hospital’s EMR or software platforms such the Philips Capsule Medical Device Information Platform (MDIP). A DDI standardizes communication so that information can flow reliably and securely from the medical device in a format that the other systems can understand. In this way, DDIs allow systems, such as the Philips Capsule MDIP, to communicate with medical devices without having to know specific operational details about the medical device hardware.
A more robust DDI library means greater ability to communicate – and integrate data – with a wider array of medical devices and manufacturers. Because Philips Capsule has been building its driver library for more than 25 years, we’ve built a DDI database with:
- Vendor-neutral interoperability: A single interface can connect multiple devices from a manufacturer to various downstream systems.
- Extensive device coverage: 250+ drivers supporting more than 1,200 unique devices.
- Scalable integration: Developed in collaboration with 150+ manufacturers to support broad, future-ready connectivity.
The Challenge of Scaling Connectivity in a Fragmented Device Market
The medical device landscape is exploding. According to the World Health Organization, there are now over two million different medical devices on the market.2 Each device type may report data differently, using unique formats, naming conventions, or communication protocols.
At the same time, downstream systems like EMRs are demanding more data, at higher levels of fidelity, and with greater consistency. When device data is not standardized, integration becomes a major operational hurdle, sometimes requiring thousands of custom transformation rules to make the data usable by another system, such an EMR. Even with broad DDI support, many hospitals face persistent pain points:
- Fragmented or inconsistent data — Each medical device may report values differently and at different frequencies
- Complex, brittle transformation logic that burdens IT teams
- High-stakes risks when data inconsistencies impact clinical decisions
A fragmented device manufacturing market and increasingly complex requirements from downstream, data-consuming systems call for a smarter, more flexible model for liberating, aggregating and sharing data than standard, one-to-one driver interfaces. This need is what led Philips Capsule to develop the Enhanced Data Model (EDM).
Introducing the Enhanced Data Model
EDM is an evolution of our DDI framework that enables the Philips Capsule MDIP to capture cleaner, richer, and more standardized data from connected devices and share it more efficiently with receiving systems downstream. DDIs that use this new model enable faster interoperability at the driver level and reduce the work required within MDIP to turn raw device data into clinically meaningful insights. With EDM, integration begins within the driver, so that the MDIP can do its work of aggregating, applying analytical rules and sharing clinical insights even faster.
How does EDM benefit data integration? EDM is:
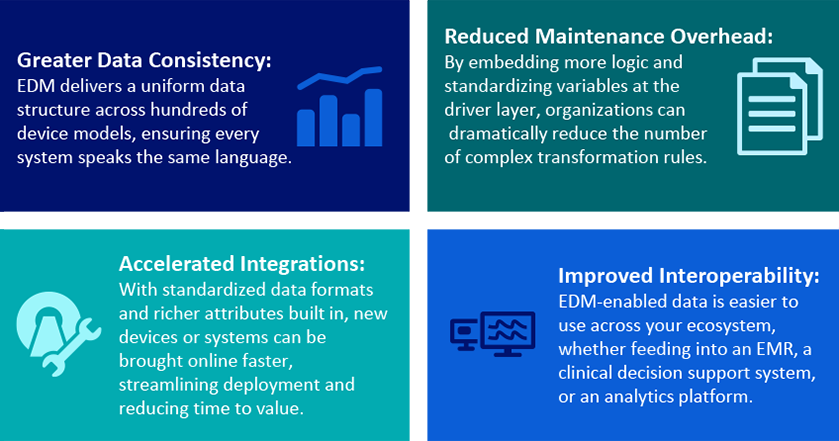
- Cleaner: EDM delivers a more uniform data structure across hundreds of device models, ensuring every system speaks the same language, delivering consistent communication across devices.
- Richer: Adds fourteen new clinically relevant attributes, including alarm priority, alarm type, alarm source, physiological minimum, and physiological maximum.
- More Standardized: By embedding more logic into the driver layer, EDM significantly reduces the need for complex, downstream rules, reduces variability and streamlines configuration. A Boston-based hospital system recently showed it could cut its transformation rules from 8,000 to just 500 (a 16-fold reduction) thanks to EDM’s standardization. Less time spent managing custom rules means more time focused on care delivery.
- Actionable: With standardized data formats and richer attributes built in at the driver level, new devices or systems can be brought online faster, streamlining deployment and reducing time-to-value. With more standardization, EDM helps prepare data faster for use by EMRs, surveillance systems, and analytics platforms with minimal transformation.
These advancements translate into material improvements across the care environment. By embedding richer, more standardized data at the driver level, EDM-enabled DDIs unlock a host of operational and clinical benefits that simplify integration and elevate data quality. EDM-enabled data is easier to use across a health system’s ecosystem, whether data is feeding into an EMR, central station, clinical decision support system, or an analytics platform.
How to Get Started
For current Philips MDI customers: The best part? EDM-enabled drivers are designed to work in parallel with your current drivers. They are fully backwards compatible, meaning you can download and adopt new, EDM-enhanced DDIs incrementally without disrupting your existing MDIP workflows or integrations. Visit the Customer Portal’s Downloads section to access the latest EDM-enabled drivers. New EDM-enabled drivers are being added daily.
For new customers: EDM is built into Philips Capsule’s MDI solution by design. That means you will get all the benefits of EDM-enabled drivers and standardized, high-fidelity data right out of the gate. From your very first deployment, you will be working with a smarter, more scalable integration model that simplifies configuration and accelerates time-to-value.
We would like to hear from you! Let us know what you are thinking and what is working, or not working, with your medical device connectivity. Please feel free to reach out at MDIProdMgmt@philips.com to share your thoughts and explore how our team can support your integration goals.
About the author
Noah Miller is a Senior Product Manager of Platform and Data Integration at Philips Capsule.
Learn more about device connectivity and Philips Medical Device Integration.
Download