Die neueste Version von Philips Capsule Surveillance erhält die FDA-Zulassung.

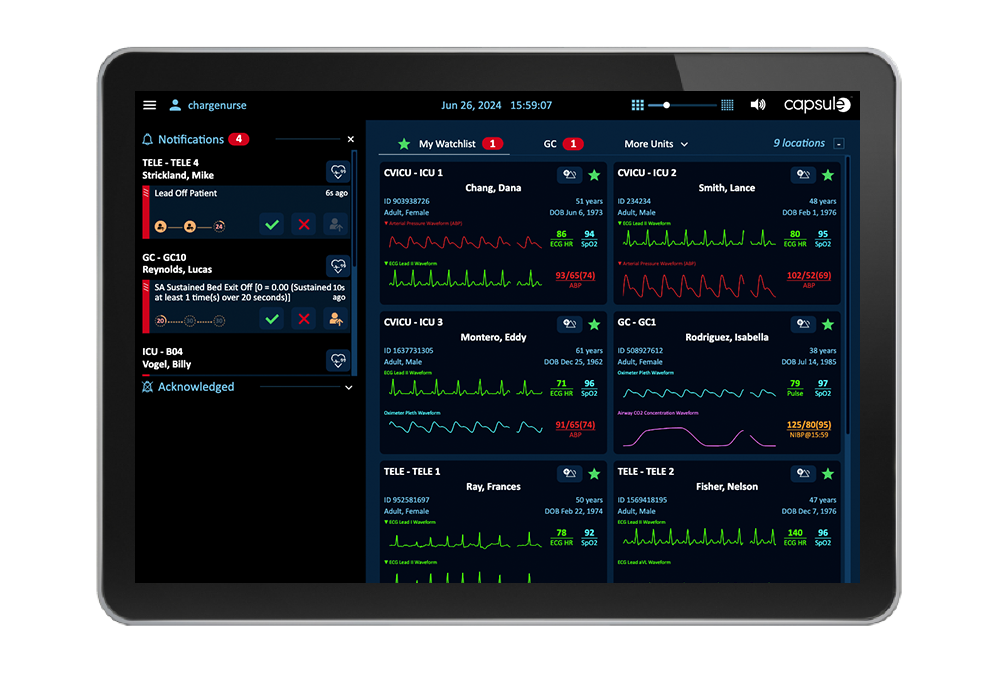
Wichtige Warnmeldungen und Alarme aller patientennahen Geräte und Systeme werden priorisiert an das zuständige Behandlungsteam weitergeleitet. Um die Anzahl der weitergeleiteten Warnmeldungen zu reduzieren und so eine Alarmmüdigkeit zu vermeiden, werden diese gefiltert.
Eine maßgeschneiderte Lösung zur Unterstützung von speziell ausgebildeten Pflegekräften, um sicherzustellen, dass sie Alarme und Warnmeldungen von Spezialgeräten erhalten und Patientendaten im Live-Stream einsehen können, wenn sie nicht am Krankenbett sein können.

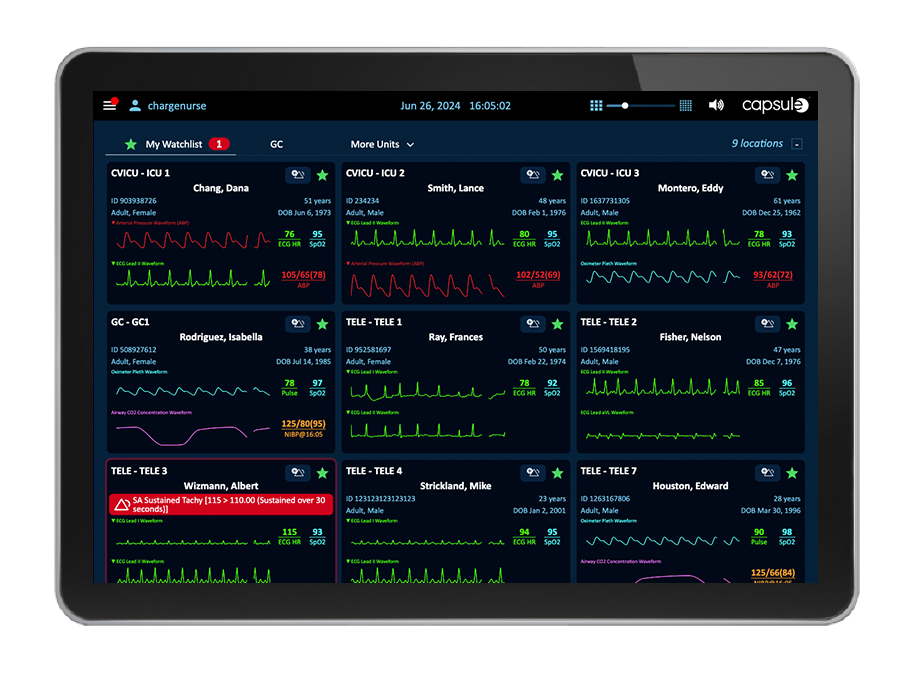
Die Transparenz aller Daten, Alarme und Warnmeldungen von patientennahen Geräten und Systemen ist entscheidend, damit sich die Pflegekräfte aus der Ferne ein Bild vom klinischen Zustand des Patienten machen können, auch wenn sie nicht am Krankenbett sind.

Unterstützung von Pflegekräften in Regionen mit niedrigem Verhältnis von Pflegekräften zu Patienten durch eine Methode, um mögliche unbemerkte Verschlechterungen zu erkennen und frühzeitig auf Anzeichen von Atemnot aufmerksam zu werden.

Unterstützen Sie den Telemetrietechniker in der zentralen Überwachungseinheit mit einer ganzheitlichen Visualisierung aller Geräte- und Wellenformdaten, verbesserter Transparenz und Verwaltung der Alarmeskalation, manuellen Alarmierungsfunktionen sowie der Möglichkeit zur EKG-Streifenannotation und zum Export.
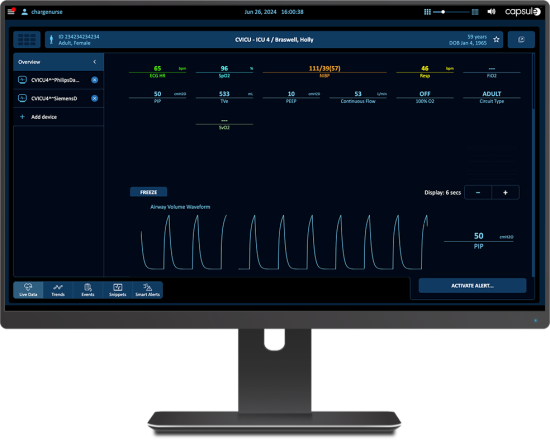
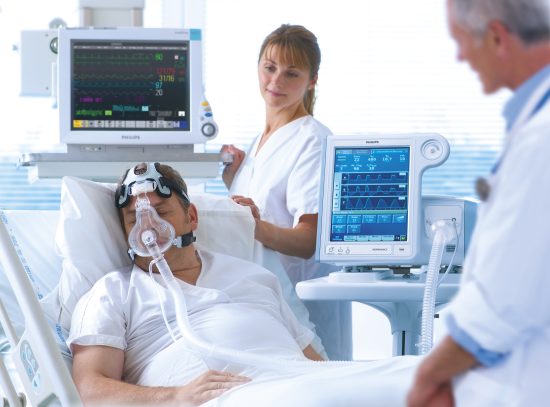
Die kontinuierliche klinische Überwachung kann dazu beitragen, den Einfluss des medizinischen Personals auf alle Intensivpatienten auszuweiten, unabhängig davon, wo diese innerhalb der Einrichtung behandelt werden. Die Überwachung beatmeter Patienten bietet eine zentrale Übersicht über die Beatmungsdaten und meldet klinisch relevante Notfälle.
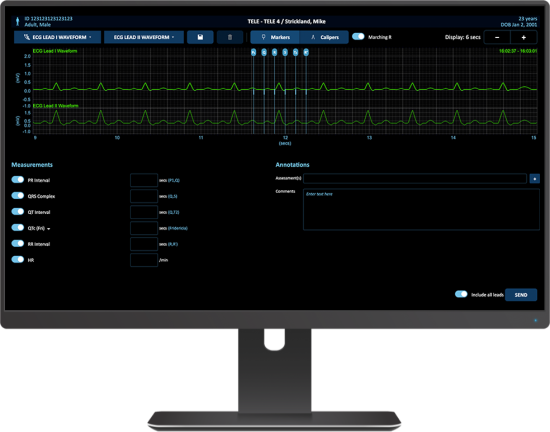
Capsule Surveillance bietet umfassende Unterstützung für die zentrale Telemetrie-Überwachungseinheit (CMU) und die damit verbundenen Arbeitsabläufe des Telemetrietechnikers. Dies geschieht durch die Bereitstellung einer ganzheitlichen Übersicht über alle Geräte- und Wellenformdaten, eine verbesserte Sichtbarkeit und Verwaltung von Alarmeskalationen, manuelle Alarmierungsfunktionen sowie die Annotation und den Export von EKG-Streifen.

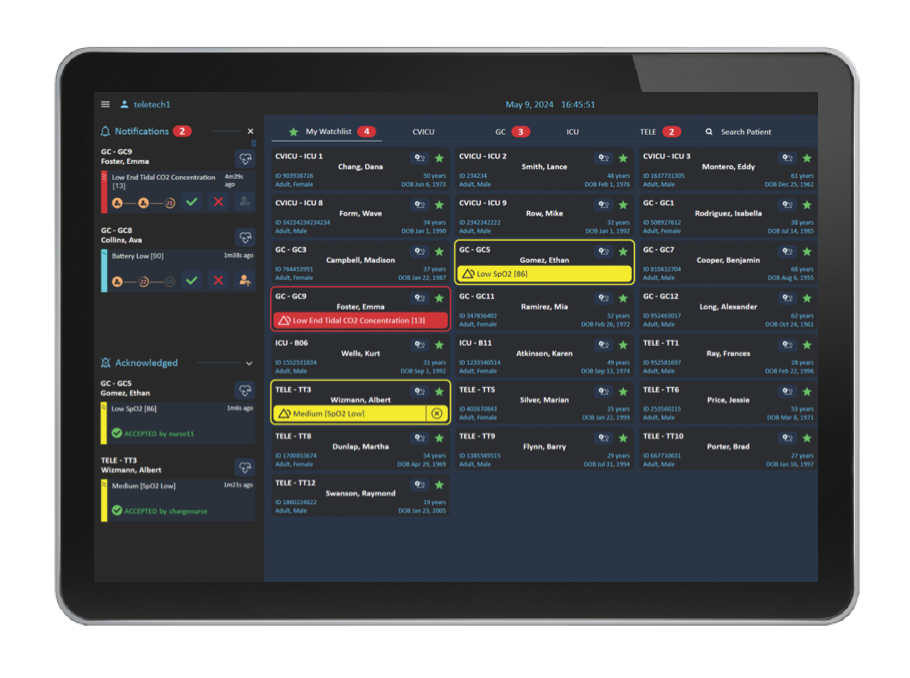
Telemetrietechniker müssen sicherstellen, dass klinisch relevante Warnmeldungen an die Pflegekräfte vor Ort weitergeleitet werden. Mithilfe des Warnmeldungs-Management-Dashboards können sie Eskalationen von Warnmeldungen verfolgen und diese bei Bedarf eskalieren oder manuell generieren. Alle relevanten Informationen können zentral abgerufen, eskaliert oder manuell generiert werden.
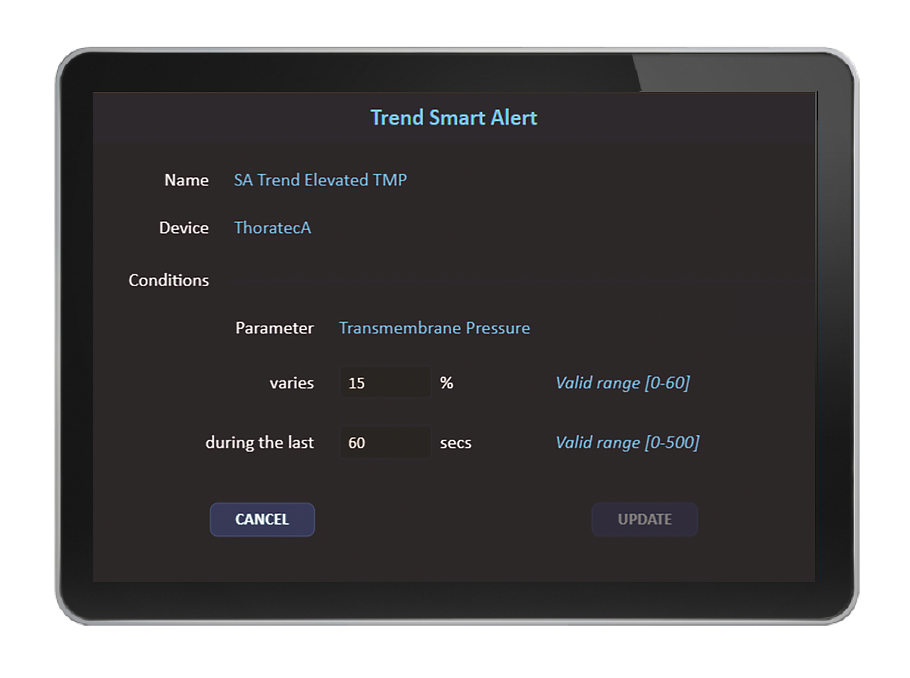
Im Rahmen der Implementierung können Capsule-Spezialisten gemeinsam mit dem Klinikpersonal intelligente Regeln für die Einrichtung und spezifische Pflegebereiche konfigurieren. Die Capsule-Überwachung ermöglicht die Anpassung der Regeln an die Präferenzen und Vorgehensweisen der Kliniker. Bestimmte intelligente Capsule-Regeln unterstützen etablierte, veröffentlichte Leitlinien für die klinische Praxis.
Mithilfe intelligenter Regeln führt die Überwachungslösung Capsule eine multivariate Beurteilung des Patientenzustands durch und benachrichtigt das Pflegepersonal mit intelligenten Warnmeldungen, sobald sich der Zustand des Patienten verschlechtert und potenziell zu Notfällen führen kann. Durch die Verteilung dieser Warnmeldungen an die Mobilgeräte oder andere Bildschirme der zuständigen Teammitglieder stehen diese dem Pflegepersonal auch unterwegs oder in Bereichen mit zusätzlicher Überwachung, wie z. B. auf einer elektronischen Intensivstation, in der Telemedizin oder in einer zentralen Überwachungseinheit, zur Verfügung.